Supercritical CO2 Solubility
Supercritical CO2 extraction process depends by CO2 solubility. Particularly is “geometrically variable”. A supercritical fluid is any compound at a temperature and pressure above its Critical Point.
It can diffuse through solids like a gas, and it can dissolve materials like a liquid. For any pure compound, there is a transition state called “critical” state: for temperatures below the critical temperature Tc two phases – liquid and vapor – coexist; for temperature above Tc, there is only one phase: supercritical fluid. Solubility is a function of pressure and temperature:
Solubility Increases with increasing pressure at constant temperature.
Solubility May Increase, or Decrease, when temperatures are raised at constant pressure.
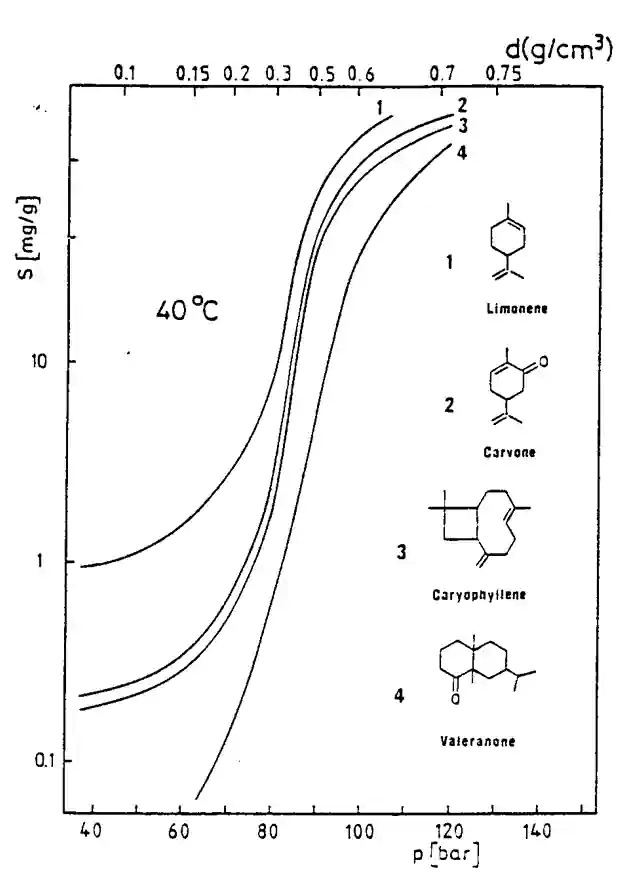
Solubility is related to density. Higher Density, Higher Solubility. This is true from the theoretical point of view, but when applied to singular compound we may see different results, as shown in the graphs below.
CO2 solubility
for “simple” systems and relatively low solubility, the empirical correlation proposed by Chrastil can be used to interpret experimental results with a good reliability without any complicated calculations :
C = density^k × exp [a/T + b]
where C is the solute concentration, a, b and k empirical constants ; this correlation shows the extreme dependence of the solubility to the fluid specific gravity. It also shows that:
- Solubility increases with density (or pressure) at constant temperature ;
- Solubility may increase or decrease when temperature is raised at constant pressure.
In all cases, the solubility dramatically decreases when the fluid is depressurized at constant temperature below its critical pressure, with solubility variation of several orders of magnitude. This is the basis of most SCF processes : SCF are used as solvents in the supercritical fluid region to selectively extract some compound(s) before being depressurized to cause the solute(s) precipitation permitting the fluid-solute separation.